
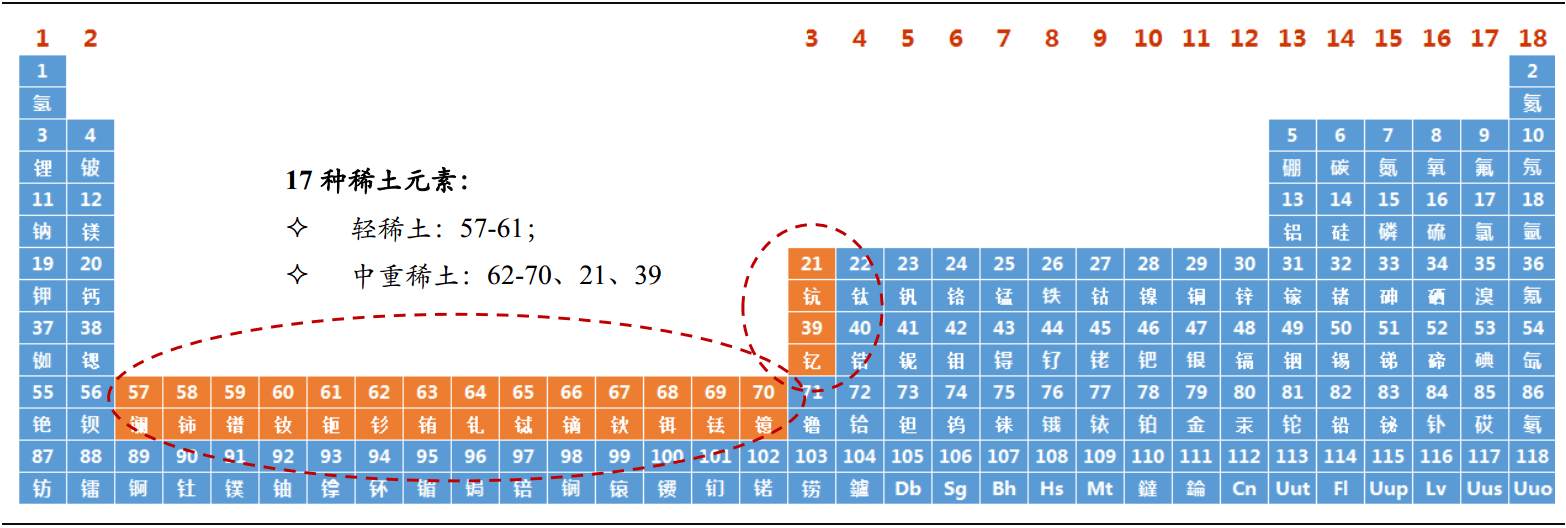
Lanthanide and its associated and similar elements in the periodic table of elements are collectively referred to as La, CE, PR, Nd, PM, SM, EU, Gd, TB, Dy, Ho, er, TM, Yb, Lu, and 17 elements closely related to 15 lanthanide elements SC and y, which are called rare earth elements (RA) Re earth (RE).
Rare earth elements were first discovered from the relatively rare minerals produced in Sweden. According to the custom at that time, "Earth" was said to be insoluble in water, so it was called rare earth.
The chemical properties of rare earth metals are very similar, so they coexist in minerals. However, the chemical properties of scandium are quite different from other rare earth metals. Generally, there is no scandium in rare earth minerals. The rarest PM was initially obtained from fission products of uranium reactors. The half-life of 147Pm is 2.7 years. In the past, it was thought that promethium did not exist in nature until 1965, when a phosphate factory in Finland found trace amounts of promethium when processing apatite.
According to the electronic layer structure and physicochemical properties of rare earth elements, their paragenesis in minerals and different ion radii, 17 kinds of rare earth elements are usually divided into two groups.
Light rare earth elements (also called Cerium Group) include lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium and gadolinium.
Heavy rare earth elements (also known as yttrium group) include terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, scandium and yttrium.
Cerium group or yttrium group is named because they are more in the rare earth mixture obtained by mineral separation.